Dominique Ebbenga, S.J. Wold-Burkness, E.C. Burkness, & W.D. Hutchison
Department of Entomology, University of Minnesota
Introduction

The Japanese beetle (Popillia japonica) (Fig. 1) is an invasive species first detected in America in 1916 in New Jersey after an accidental introduction. As suggested, the pest is native to Japan, where it is regarded as a minor agricultural pest. Since its arrival, populations have fluctuated from year to year. However, populations have increased considerably during the past 5 years; recent research documented, in high density years that traps can catch >5,000 beetles per day in southern Minnesota. The Minnesota Department of Agriculture maintains a state map, illustrating the spread of Japanese beetle within the state (see Related References).
Identification
Adult Japanese beetle can be identified by a metallic green head and thorax, copper brown wing covers, five white hair tufts on the side of their abdomen, and two tufts on the rear of their abdomen (Fig. 2). Their size ranges between 1/3 to 1/2 inch in length. The white tufts of hair distinguish them from the sand chafer or false Japanese beetle as the sand chafer does not have the hair tufts but otherwise look very similar in size and shape (Fig. 3).


Larvae, also known as white grubs, are found in the soil where they feed on grass roots. They are C-shaped, with a white to cream colored body, tan head capsule, and covered in small brown hairs (Fig. 4). Depending on the larval stage, they can be anywhere from 1/8 to 1 inch in length. They can be very difficult to distinguish from other grub species found in the soil. Proper identification of Japanese beetle larvae requires looking at raster patterns and slit shapes on the rear of the insect (Fig. 5).


Life Cycle
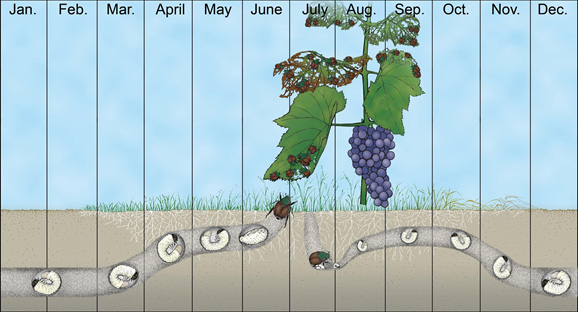
Japanese beetle overwinters below the soil surface as larvae between 1 to 6 inches deep (Figure 6). Once spring begins, the larvae begin to move up to the soil surface, usually within the top 2 inches, to resume feeding on grass roots until they reach pupation. Once they pupate, adults will begin emerging from the soil at the end of June to early July, and disperse to adjacent or nearby host plants to feed, including ornamental and agricultural crops. Soon after adult emergence, and while actively feeding, mating also begins. Females will then move from host plants to areas of sod or other grasses to lay eggs, in clusters, beneath the soil surface. Following egg hatch, larvae will undergo three larval stages (instars) and remain in the soil, fall to spring; as they mature during autumn, the grubs will burrow deeper into the soil in preparation to overwinter.
Damage
Damage by Japanese beetle can be caused by both adults and larvae. Adults feed on the above ground plant parts, including flowers, leaves and fruit of >300 plant species. However, the larval stage feeds exclusively on grass roots. The timing of damage by both adults and larvae closely follows the life cycle phenology as illustrated in Fig. 6.
Preferred fruit crops include grapes (including wild grape), raspberries, and apples. In addition, many ornamental plants are impacted, including rose, linden, and crab apple. When adults feed on crop foliage, they create a distinctive pattern of defoliation on the leaf known as “skeletonization” (Fig. 7). They do this by eating the tissue in between leaf veins. In some cases, leaves can become severely defoliated, turn brown, and drop off the plant, reducing photosynthesis. As beetles feed chemical volatiles (signals) are released which attract other beetles to the crop (Figs. 8-10). Feeding will usually begin at the top of the canopy on new foliage and may eventually move to older foliage when populations are high.
Adult Japanese beetle damage can be alarming when beetle density is high and can lead to severe defoliation. However, unlike field crops (corn, soybean), very little information is available regarding the potential risk of adult defoliation on fruit crops. Research is currently underway at the UMN to quantify the potential impact of adult feeding on yields of fall raspberry and wine grapes. For perennial crops such as grapes and raspberries, older, well established plants appear to be able to recover or compensate from feeding damage, but long-term effects are unknown. However, young, newly established plantings may not tolerate much feeding, resulting in stunted growth if feeding damage is severe. As populations continue to increase and spread across Minnesota, it is important to determine the potential economic impact this pest could have in the future.



Adults prefer to lay eggs in grassy areas and therefore are typically found feeding on grass roots. Egg lay occurs from August-November, and surviving overwintering larvae feed on grass roots from April to the end of May. Larvae damage grass by pruning off the roots which reduces water uptake. This feeding damage can result in brown patches of sod that easily lift up from the ground when larval densities are high.
Management
Adults
Due to the lack of research on the impact of Japanese beetle feeding on the yield and quality of fruit crops, economic thresholds have not been established for this pest. However, we have developed a preliminary forecasting model based on the well-known "growing degree-days" concept, used for predicting insect and plant growth under field conditions, to improve advance notice of JB infestations. For more information on our ongoing research with JB degree-days see the UMN Extension article.
If populations are high and growers are concerned about excessive defoliation in a crop, the use of insecticides, such as carbamates and pyrethroids, or organic options such as Entrust or Pyganic can be effective management tools. Always be sure to read labels carefully and follow recommended rates for application.
Alternative management options have not been studied extensively, however, the use of physical barriers, such as exclusion netting or high tunnels, may be used to prevent, delay or suppress beetle populations. Preliminary data from UMN research suggests that high tunnels configured with fine mesh netting, or poly over the top (with open ends) can be very effective at managing adult beetles. This strategy has the added benefit of reducing insecticide use.
In its native range, biological control agents have been a major help in reducing the pest status of this insect. However, when Japanese beetle was introduced to the U.S., these biological control agents were not present to help manage population levels. One biological control agent that has become more noticeable in the past 4 years in Minnesota, is the winsome fly (Istocheta aldrichi). The fly is conspicuous as it lays its white eggs just behind the head of adult Japanese beetles (Figs. 11 and 12). Once eggs hatch, larvae burrow into the host and feed, eventually killing the beetle within 7-10 days. One advantage for adult control is that recent observations (W. Hutchison) indicate that the parasitoid often prefers to lay eggs on female beetles; long-term this should help suppress local populations via reduced egg-lay at the population level. Although parasitism rates have been historically low in Minnesota (<5%), several samples in 2023 confirmed 20-25% parasitism. This outcome is encouraging given the severe winter weather this past year, and that the parasitoid may now be better adapted to the Minnesota climate (southern MN), and the fly's emergence phenology better aligned with that of Japanese beetle emergence. Continued monitoring and studies on this biological control agent will help to better understand the potential of this being a feasible control option in the future.


Larvae
Typically, larval control with soil insecticides is pursued with the intent of reducing infestations the following season, i.e. applications are completed in August-September when most larvae are still in the early instar stage and in the top 3 inches of soil. Thus, when controlling larvae, it is important to keep in mind this will not have an impact on the current season's adult population. Also, it is important to keep in mind that beetles can disperse from any given farm from surrounding locations. Therefore, controlling the larval stage may not be effective if neighboring areas are not treated. Larval management focuses on turf areas where eggs are laid, and larvae develop. Applications of conventional insecticides or biologicals such as Bacillus thuringiensis can be made to turf in late August to early September (see Fig. 2) to control larvae in the early growth stages and before they move too deep in the soil.
References
Burkness, E.C., D.N. Ebbenga, & W.D. Hutchison. 2020. Evaluation of Foliar Insecticide Control of Adult Japanese Beetle in Raspberry—2019. Arthropod Mgmt. Tests. 45: Issue 1, January 2020, tsaa009; https://doi.org/10.1093/amt/tsaa009
Ebbenga, D., Burkness, E., Tonito, A., and W.D. Hutchison. 2021. New Forecasting Model for Japanese Beetle in Minnesota. https://blog-fruit-vegetable-ipm.extension.umn.edu/2021/04/new-forecasting-model-for-japanese.html
Hahn, J. & J. Weisenhorn. 2018. Japanese Beetle in Yards and Gardens. https://extension.umn.edu/yard-and-garden-insects/japanese-beetles
Japanese Beetles (E0010TURF), Davis 2018: https://www.canr.msu.edu/resources/japanese_beetles_e0010turf
MDA. 2020. Japanese beetle map, MN; https://www.mda.state.mn.us/plants-insects/japanese-beetle
Shanovich, H.N. et al. 2019. Biology and Management of Japanese Beetle (Coleoptera: Scarabaeidae) in Corn and Soybean: https://academic.oup.com/jipm/article/10/1/9/5454734
Potter, D.A. & D.W. Held. 2002. Biology and Management of the Japanese Beetle: https://www.annualreviews.org/doi/full/10.1146/annurev.ento.47.091201.145153
Fleming, W.E. 1972. Biology of the Japanese Beetle: https://naldc.nal.usda.gov/download/CAT87201410/PDF
Updated July, 2023